Translate this page into:
Screening of Hearing Impairment in High-Risk Neonates: A Study at Dr. R.N. Cooper Hospital and H.B.T. Medical College
Address for correspondence Megha Mukund Panjiyar, MBBS, DNB (ENT), Department of Otolaryngology, Dr. R.N. Cooper Hospital and H.B.T. Medical College, E-703, Ashtvinayak CHS, Moongipa Arcade, D N Nagar, Andheri (W), Near Ganesh Chowk, Mumbai 400053, Maharashtra, India (e-mail: megha.pd@gmail.com).
Abstract
Introduction
The aim of this study was to find out the prevalence of hearing impairment in high-risk neonates born in Dr. R.N. Cooper Hospital and H.B.T Medical College and establish the fact that high-risk neonates have higher prevalence of hearing impairment compared with normal population.
Materials and Methods
A prospective observational study was conducted over a period of 1 year starting from March 2016 and involved three-stage screening of 410 neonates admitted to neonatal intensive care unit of Dr. R.N. Cooper Municipal General Hospital and H.B.T Medical College, Vile Parle (west), Mumbai. All enrolled neonateswere screened by a three-stage screening mechanism. First otoacoustic emission (OAE-1) screening was done within 24 to 72 hours of birth. Parents of neonates referred by OAE-1 were instructed to come back within 28 days for repeat OAE test (OAE-2). Those referred by OAE-2 were asked to come back after further 2 months for brainstem-evoked response audiometry. Data collected in the study were processed using Microsoft Excel.
Results and Conclusion
In the current study, out of 410 neonates who were screened by a three-stage screening mechanism, five including three girls and two boys were found to be suffering from profound sensorineural hearing loss. Observed prevalence of 12.20 (or 12 on rounding off to nearest digit) per 1000 in high-risk neonates is much higher compared with the prevalence of 1 to 6 per 1000 live births in overall population as reported by the American Speech-Language-Hearing Association.
Keywords
high-risk neonates
prevalence of hearing impairment
SNHL
Introduction
The cause of sensorineural hearing loss (SNHL) can be broadly classified as genetic, acquired, or idiopathic, wherein genetic causes alone lead to ~50% of moderate-to-profound congenital SNHL.1,2 Only 15 to 30% of cases are associated with named syndromes or other anomalies (nonsyndromic).3 Furthermore, 80% cases are transmitted in an autosomal-recessive pattern, whereas the remaining are autosomal-dominant, mitochondrial, or sex-linked.4
Nearly 600 syndromes and 125 genes associated with hearing loss have already been identified. Joint Committee on Infant Hearing, the American Speech-Language-Hearing Association suggests that hearing evaluation should include a review of family history of specific genetic disorders or syndromes, including genetic testing for gene mutations and syndromes commonly associated with earlyonset childhood SNHL.5
American Academy of Pediatrics Joint Committee on Infant Hearing issues position statement in 2007 stating recommendations for newborn hearing screening. It recommends screening for low-risk neonates by otoacoustic emission (OAE) and for high-risk neonates by brainstem response testing within the first month of birth. Neonates failing initial screening to be sent for repeat testingand neonates failing both the screening to be sent for comprehensive audiometric evaluation by 3 months of age.5
Both computed tomography (CT) and magnetic resonance imaging (MRI) are integral part of overall assessment of children with SNHL. High-resolution CT of temporal bone is more rapid, requires less time, and costs less than MRI, whereas MRI is advantageous in identifying cochlear nerve anomalies and is also associated with smaller dose of ionizing radiation.6
Failure to detect and effectively manage hearing impairment in the first 6 months of life has been associated with substantial and irreversible deficits in speech, linguistic, and cognitive development, which can result in poor educational and vocational attainment in later life. If not screened at the time of birth, by the time hearing loss in early childhood is suspected, audiologically evaluated, and appropriately managed two or more of these critical years are missed and the child loses an enormous developmental advantage.
Adults with hearing impairment also have a much higher unemployment rate and among those employed are majorly in low-grade employment. The onus lies on modern medical system to develop culturally and financially acceptable ways of implementing infant hearing screening programs.
In a developing country like India, healthcare system is yet to evolve to act effectively on the preventable cause. Findings of a study done by Kumar and Mohapatra, summarized in ►Table 1, show that only 38.09% of the medical colleges have universal newborn hearing screening program in comparison to 80% of the speech and hearing centers.7
| Types of institutions | No. of institutions | Institutions with NBHS program | Institutions without NBHS program |
|---|---|---|---|
| Medical colleges | 21 | 8 (38.09%) | 13 (61.90%) |
| Speech and hearing centers | 10 | 8 (80%) | 2 (20%) |
Abbreviation: NBHS, newborn hearing screening.
The prevalence of congenital hearing loss has been reported to be 1 to 6 per thousand live births by the American Speech-Language-Hearing Association.1 A study at tertiary care hospital by Jose et al at Trivandrum, Kerala, found manifold prevalence of hearing impairment among high-risk neonates at 9 per thousand live births.8
Studies also indicate prevalence of hearing impairment among high-risk neonates is much higher than normal neonates. Referring neonates at high risk, such as those with a family history of deafness or those born with low birth weight, birth asphyxia, jaundice, or meningitis, for early assessment of hearing to ensure prompt diagnosis and appropriate management is necessary.
Need of Study: Importance of Early Detection
The 2011 census data reveal that 26.8 million or 2.13% of total Indian population suffers from some kind of disability. Hearing represents the second highest fraction of the disability at 19% together with disability in vision as shown in ►Fig. 1. The share of hearing disability among age group 0 to 19 is even more profound at 20%.9
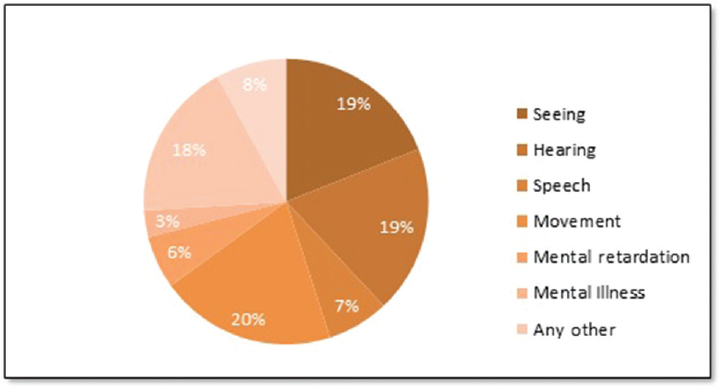
- Proportion of disability in India.9
A study done by Gulati et al, on 14,123 neonates born at Lok Nayak Hospital, Delhi, in the period from February 2013 to September 2014 found drastic difference in prevalence of hearing impairment among normal neonates and high-risk neonates. Of the 14,123 neonates, 64 were neonatal intensive care unit (NICU) neonates categorized as high-risk neonates. Through a three-stage screening study, 44 of the 14,059 normal neonates were found suffering from hearing loss, representing a prevalence of 3.12 per thousand. In contrast, 64 NICU neonates were made to undergo brainstem-evoked response audiometry (BERA) test irrespective of whether they passed or failed OAE. A total of three neonates of these 64 NICU infants were found to suffer from hearing loss representing prevalence at staggering 46.9 per thousand.10
Another study by Vashistha et al, conducted on 100 high- risk neonates at J.L.N. Medical College, Ajmer, between 2013 and 2014, found a staggering prevalence of unilateral and bilateral hearing loss of 150 per thousand.11
A study by Regina et al on 613 high-risk neonates discharged from NICU of Academy of Medical Sciences, Kannur, Kerala, and Sri Siddhartha Medical College and Research Centre, Tumakuru, Karnataka, between August 2015 and August 2016 based on behavioral audiometry, OAE, and auditory brainstem response (ABR) found 42 cases of hearing impairment, representing prevalence of 67.6 per 1,000 neonates.12
Study on neonates admitted to the NICU at Nemazee Hospital, Shiraz University of Medical Sciences, between January 2006 and January 2007 shows higher prevalence of hearing loss in newborns with a gestational age of less than 36 weeks. The study also concludes that prematurity should be considered as a risk factor for hearing loss as these neonates face problems due to the underdevelopment of their respiratory system, which requires more and longer oxygen therapy, and as a consequence of their weakened immune system, they are also exposed to various infections and thus the chance of sepsis and the use of antibiotic therapy will be higher.13
Study on guinea pigs by Haupt et al showed changes in cochlear microcirculation and oxygenation and auditory function during prolonged hypoxic ventilation. The study recorded mean reduction in cochlear and brainstem potentials to 75 to 82%.14
In a study done at Ahmadi Hospital by Al-Kandari and Alshuaib, in Kuwait, hearing loss in the well infant group was found at 2%, while in the high-risk group it was found whooping 46.67%. Study was performed on 200 normal (“well baby”) newborn children and 15 children at high risk for hearing loss at Ahmadi Hospital in Kuwait. Distortion product otoacoustic emissions (DPOAEs) were conducted in the ward on newborns at the age of 2 days. Newborns who did not pass the DPOAE the second time (at the age of 1 month) were evaluated by a BERA test within the age of 3 months. If the infant failed the BERA test, the test was repeated at the age of 6 months to confirm any permanent hearing loss. The incidence of permanent hearing loss in the “well infant” group was 2%, and 98% had normal hearing level. In the “high-risk” group, 46.67% had permanent hearing loss of which 26.67% had profound SNHL and 20% had moderate (60 dBnHL) SNHL, and 53.33% had normal hearing level.15
The prevalence of congenital hearing loss has been reported to be 1 to 6 per thousand live births by the American Speech-Language-Hearing Association.6 Another study at tertiary care hospital by Jose et al at Trivandrum, Kerala, found manifold prevalence of hearing impairment among high-risk neonates at 9 per thousand live births.8
Another study by Dr. D M Ambekar of Department of ENT, Terna Medical College, Nerul, Navi Mumbai found referral rate for BERA after first stage DPOAE screening of 12% which got reduced to 1% after second stage DPOAE screening. The two- stage DPOAE screening results indicated hearing loss at 0.36% in no risk group and 7.69% in high-risk group. The study also concluded the need of two-stage OAE screening as it reduces referral rates drastically. In a highly populated and developing country like India, two-stage OAE screening is cost-effective and reduces the referral rates for BERA.16
A study done by Kumar et al concluded the need for universal screening test and not to screen just high-risk neonates. The study found that the two-stage hearing screening with transitory-evoked OAE and ABR is a feasible method that can be successfully implemented for newborn hearing screening, for early detection of hearing impaired, on a large scale. Of the 800 newborns screened, total five were found to be suffering from hearing impairment. Three of the five hearing impaired detected in the study had no known risk factor for hearing loss, advocating need for universal hearing screening.17
A study done at CMC Vellore on 500 neonates reported 6.4 and 1.6% hearing loss at initial and repeat screen with DPOAE. It further concluded that screening done by DPOAE followed by BERA would minimize referral rates.18
Another study during 2007 to 2008 at Union Hospital and Queen Elizabeth Hospital, Hong Kong found staged DPOAE and automated ABR screening had similar final referral rate as ABR-only screening protocol, but it was ~2.5 times cheaper and almost three times faster.19
Many factors play a role in placing NICU neonates at an increased risk of hearing loss, including underlying disease processes as well as the treatment they receive. Periods of profound hypoxia-ischemia, treatment of neonates with respiratory failure by hyperventilation or alkalizing therapy, might compromise the oxygenation and perfusion of the cochlear organ and auditory pathway. The use of ototoxic drugs, including loop diuretics and aminoglycosides, has been associated with increased vulnerability of the cochlea to damage from pre-existing hypoxia.
Study Population
The study population comprised of all the high-risk neonates born in H.B.T Medical College Dr. R. N. Cooper Municipal General Hospital and admitted in NICU during the study period that qualified the inclusion and exclusion criteria mentioned in following sections.
Sample Size
For our study, we enrolled a total of 478 neonates admitted in NICU, but out of these 68 neonates were either transferred to higher center or took discharge against medical advice. A total of 410 neonates completed the study. Additional 21 eligible neonates died in NICU during the study.
Time Frame to Address the Study
Neonates admitted to NICU between March 2016 and February 2017 were selected for the study. BERA was performed after 3 months of doing the OAE-1. Accordingly, neonates born in February were taken for BERA in the month of May 2017.
Inclusion Criteria
Joint Committee on Infant Hearing, American Speech-Language-Hearing Association suggests that the use of risk indicators for identifying neonates who should receive audiological evaluation but who live in developing nations or remote areas where universal hearing screening is not yet available. The committee lists 11 risk indicators that are associated with either congenital or delayed-onset hearing loss.5 These are as follows:
Caregiver concern regarding hearing, speech, language, or developmental delay
Family history of permanent childhood hearing loss
Neonatal intensive care of more than 5 days or any of the following regardless of length of stay: extracorporeal membrane oxygenation, assisted ventilation, exposure to ototoxic medications (gentamycin and tobramycin), or loop diuretics (furosemide/Lasix), and hyper bilirubinemia that requires exchange transfusion.
In utero infections, such as cytomegalovirus, herpes, rubella, syphilis, and toxoplasmosis
Craniofacial anomalies, including those that involve the pinna, ear canal, ear tags, ear pits, and temporal bone anomalies
Physical findings, such as white forelock, that are associated with a syndrome known to include a sensorineural or permanent conductive hearing loss
Syndromes associated with hearing loss or progressive or late-onset hearing loss, such as neurofibromatosis, osteopetrosis, and Usher syndrome; other frequently identified syndromes include Waardenburg, Alport, Pendred, and Jervell, and Lange-Nielson
Neurodegenerative disorders, such as Hunter syndrome, or sensory motor neuropathies, such as Friedreich ataxia and Charcot-Marie-Tooth syndrome
Culture-positive postnatal infections associated with SNHL, including confirmed bacterial and viral (especially herpes viruses and varicella) meningitis
Head trauma, especially basal skull/temporal bone fractures, that requires hospitalization
Chemotherapy
According to the findings and suggestions in the studies mentioned above, newborn neonates admitted to NICU falling under at least one of the risk factors listed below were included in the study:
Birth asphyxia
Condition of labor and delivery (leaking per vaginum [PV], meconium-stained liquor [MSL], lower segment cesarean section [LSCS], birth trauma)
Consanguineous marriage
Fetal factors (intrauterine growth restriction [IUGR], twins, oligohydramnios)
Meconium aspiration syndrome
Prematurity (<37 weeks)
Respiratory distress
Sepsis
Low/high birth weight
Congenital anomaly
Family history of congenital deafness
Maternal factors (TORCH, pregnancy-induced hypertension [PIH], gestational diabetes mellitus [GDM], Rhesus [Rh], hypothyroid)
Neonatal jaundice
Refuse feed
Respiratory distress syndrome
Exclusion Criteria
Neonate who were on ventilator support from birth to death
Active ear infections
Parents of neonates not willing to give informed consent for their neonates to participate in the study
Methodology
All eligible neonates according to the inclusion and exclusion criteria were enrolled, and informed consent for participation was taken. Screening was done for neonates by DPOAE within 72 hours of NICU admission (OAE-1). Neonates referred by OAE-1 were instructed to come back within 28 days of birth for repeat DPOAE test (OAE-2). The result “Refer” by OAE signifies that hearing impairment is probable and that other hearing tests need to be done to confirm hearing impairment and also to find out the extent of impairment. Neonates referred by OAE-2 were asked to come back after further 2 months for BERA. Detailed flowchart is shown in ►Fig. 2. ►Fig. 3 shows DPOAE test being performed during screening of an infant for the study in Dr. R.N. Cooper Hospital and H.B.T Medical College.

- Methodology for three-stage screening.

- DPOAE test on a baby during the study. BERA, brainstem- evoked response audiometry; DPOAE, distortion product otoacoustic emission.
Findings of the Study
►Table 2 lists details of risk factors of neonates found with profound SNHL by the three-stage screening process.
| Case no. | NICU stay days | Risk factors |
|---|---|---|
| 37 | 38 | NICU stay days–38, MSL |
| 109 | 9 | NICU stay says–9, LBW, birth asphyxia, LSCS, family history of congenital deafness |
| 163 | 27 | NICU stay days–27, VLBW, respiratory distress, prematurity (<37 weeks), meconium aspiration syndrome, LSCS |
| 225 | 21 | NICU stay days–21, LBW, respiratory distress syndrome, refuse feed, IUGR |
| 370 | 130 | NICU stay days–130, VLBW, birth asphyxia, neonatal jaundice |
Abbreviations: IUGR, intrauterine growth restriction; LSCS, lower segment cesarean section; NICU, neonatal intensive care unit; MSL, meconium-stained liquor; SNHL, sensorineural hearing loss; VLBW, very low birth weight.
Five neonates including three girls and two boys were found to suffer from profound SNHL after three-stage screening test. This shows prevalence of 12.20 (or 12 on rounding off to nearest digit) per thousand.
Discussion
In present study, a total of 410 neonates were screened with DPOAE within 72 hours after birth or NICU admission (OAE- 1). The first DPOAE test was done not before 24 hours as it may give higher fail results due to occlusion of external auditory canal with debris, amniotic fluid. Neonates who had failed the OAE-1 were screened again under OAE-2 within 28 days to remove the false refers of OAE-1. As a confirmatory test, neonates referred by OAE-2 were subjected to BERA after further 2 months.
Five neonates including three girls and two boys were found to be suffering from profound SNHL after three-stage screening test. This shows prevalence of 12.20 (or 12 on rounding off to nearest digit) per thousand.
Prevalence of 12 per thousand live births found in current study is much higher than the prevalence of 1 to 6 per thousand live births reported by the American Speech-Language-Hearing Association.1 This is even higher compared with the study at tertiary care hospital at Trivandrum, Kerala, which found prevalence of hearing impairment among high- risk neonates at 9 per thousand live births.8 The prevalence of 12 observed in the study falls much short of 46.9 found by Gulati et al, on their study on 14,123 neonates born at Lok Nayak Hospital.10
The prevalence of 12 per thousand is much less compared with the findings of two-stage DPOAE screening resulting in 7.69% in high-risk group. This can be easily accepted considering even the Ambekar suggested need for confirmatory test to be done after two-stage OAE screening.16
Findings of the current study are in confirmation with the findings of Pourarian et al, in his paper “Prevalence of Hearing Loss in Newborns Admitted to Neonatal Intensive Care Unit”10 and findings of Sohmer et al, in his paper on multimodality evoked potentials in hypoxaemia.20 Underlying disease of neonates as well as the treatment they receive plays important role in placing NICU neonates at an increased risk of hearing loss. Periods of profound hypoxia-ischemia, treatment of neonates with respiratory failure by hyperventilation or alkalizing therapy, compromise the oxygenation and perfusion of the cochlear organ and auditory pathway.
No money was charged for performing tests on neonates for the study but on an average market rate of doing DPOAE for a baby is around ₹ 900 while average cost of doing BERA is around ₹ 2,200. The study also found cost saving of 42% using three-stage screening over BERA only screening. In addition, there is significant time and man-power saving by doing three-stage screening over one-stage or two-stage BERA only screening. A typical OAE screening takes not more than 5 minutes, while BERA takes 45 minutes to an hour. BERA at times takes much more time and might require multiple sittings in case baby is not sleeping easily or does not remain stationary during the test period. Moreover, BERA needs specially trained and experienced personnel for operating the machine and for interpretation of test result, while OAE is an objective test, easily repeatable, reliable, and very fast test that can be performed by any trained person not necessarily a clinician or an audiologist.
Conflict of Interest
None declared.
References
- American Speech Language Hearing Association. The Prevalence and Incidence of Hearing Loss in Children 2005
- [Google Scholar]
- A stepwise approach to the diagnosis and treatment of hereditary hearing loss. Pediatr Clin North Am. 1999;46(1):35-48.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology, etiology, and genetic patterns. In: Gorlin RJ, Toriello HV, Cohen MMJ Jr, eds. Hereditary Hearing Loss and Its Syndromes. New York: Oxford University Press; 1995.
- [Google Scholar]
- Causes of pediatric sensorineural hearing loss: yesterday and today. Arch Otoloaryngol Head Neck Surg. 1999;125(5):517-521.
- [CrossRef] [PubMed] [Google Scholar]
- Year 2007 Position Statement: Principles and Guidelines for Early Hearing Detection and Intervention Programs. American Speech-Language-Hearing Association 2007
- [Google Scholar]
- Is computed tomography (CT) or magnetic resonance imaging (MRI) more useful in the evaluation of pediatric sensorineural hearing loss? Laryngoscope. 2010;120(12):2358-2359.
- [CrossRef] [PubMed] [Google Scholar]
- Status of newborn hearing screening program in India. Int J Pediatr Otorhinolaryngol. 2011;75(1):20-26.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of hearing impairment among high risk neonates- a hospital based screening study. Int J Biol Adv Res. 2016;7(3):131-134.
- [CrossRef] [Google Scholar]
- Data On Disability, Office of the Registrar General & Census Commissioner, India, Office of the Registrar General & Census Commissioner, India, Ministry of Home Affairs, Government of India. 2011
- [Google Scholar]
- Outcome of newborn hearing screening - a tertiary care centre experience. Int J Biol Med Res. 2016;7(2):5574-5581.
- [Google Scholar]
- Prevalence of hearing impairment in high risk infants. Indian J. Otolaryngol Head Neck Surg. 2016;68(2):214-217.
- [CrossRef] [PubMed] [Google Scholar]
- Audiological screening of high risk infants and prevalence of risk factors. Int J Contemp. Pediatrics. 2017;4(2):507-511.
- [CrossRef] [Google Scholar]
- Prevalence of hearing loss in newborns admitted to neonatal intensive care unit. Iran J Otorhinolaryngol. 2012;24(68):129-134.
- [Google Scholar]
- Changes in cochlear oxygenation, microcirculation and auditory function during prolonged general hypoxia. Eur Arch Otorhinolaryngol. 1993;250(7):396-400.
- [CrossRef] [Google Scholar]
- Newborn hearing screening in Kuwait. Electromyogr Clin Neurophysiol. 2007;47(6):305-313.
- [Google Scholar]
- Newborn hearing screening - need of the hour. Int J Recent Trends Sci Technol. 2016;19:109.
- [Google Scholar]
- Feasibility of two staged new-born hearing screening and identification of risk factors for hearing loss other than those included in high risk registry given by joint committee on infant hearing. I nt J Med Sci Public Health. 2014;3(3):257-260.
- [CrossRef] [Google Scholar]
- Neonatal screening for hearing loss: pilot study from a tertiary care centre. Indian J. Otolaryngol Head Neck Surg. 2009;61(1):23-26.
- [CrossRef] [PubMed] [Google Scholar]
- The Universal Neonatal Hearing Screening (UNHS) program in Hong Kong: the outcome of a combined otoacoustic emissions and automated auditory brainstem response screening protocol. HK J Paediatr. 2010;15(1):2-11.
- [Google Scholar]
- Multi-modality evoked potentials in hypoxaemia. Electroencephalogr. Clin Neurophysiol. 1986;64(4):328-333.
- [CrossRef] [PubMed] [Google Scholar]








