Translate this page into:
“A Rare Case Report on Auditory Neuropathy Spectrum Disorder in Twins”
*Corresponding author: M. Mohan Raj, Department of Otorhinolaryngology and Head Neck Surgery, Jawaharlal Nehru Hospital and Research Centre, Bhilai, Chhattisgarh, India. mohanraj0413@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Raj MM, Jaiswal AA, Satpute SS, Umredkar G. “A Rare Case Report on Auditory Neuropathy Spectrum Disorder in Twins”. Ann Otol Neurotol. 2025;6:e011. doi: 10.25259/AONO_8_2025
Abstract
Auditory Neuropathy Spectrum Disorder (ANSD) interrupts signal transmission of sound and its temporal encoding, leading to impaired speech discrimination. ANSD is diagnosed before four years of age in most cases. Cases of ANSD in twins, particularly with late presentation at 19 years of age after normal neonatal hearing screening, are rare. This case illustrates the clinical presentation, diagnosis, and management of late-onset ANSD in 19-year-old male twins, the need for increased bimodal newborn screening procedures, and the impact of delayed diagnosis on the participant’s quality of life. 19-year-old male twins born in a non-consanguineous marriage had bilateral hearing loss. Detailed history, clinical examination, audiological and radiological investigations were conducted. Pure Tone Audiometry (PTA) indicated sensorineural hearing loss in Twin A and inconsistent results in Twin B. Impedance audiometry showed type A tympanograms with absent stapedial reflexes. Passed in otoacoustic emission (OAE), but bilateral Vth peak absent in both ears at 105 decibel hearing level (dB HL) in Brainstem Evoked Response Audiometry (BERA). Hearing aids were ineffective; psychosocial support was provided, with cochlear implantation planned. This case serves as a compelling call to action for improving neonatal screening methods by compulsory dual OAE and BERA, especially for the high-risk population. Conventional hearing aids offer only limited benefits, leaving cochlear implantation as the only option. Future research on the genetics of ANSD is needed.
Keywords
Auditory neuropathy spectrum disorder
Cochlear implant
Late-onset
Neonatal screening
Twins
INTRODUCTION
Auditory Neuropathy Spectrum Disorder (ANSD) is a unique problem in the continuum of auditory disorders with preserved outer hair cell function of the cochlea juxtaposed against signal transmission defects anywhere between inner hair cell synapses and the auditory neural fibres.
About 1–3 out of every 10,000 live births are affected by ANSD, but it is much more common in children hospitalized in the neonatal intensive care unit (NICU), where it causes 10%–15% of sensorineural hearing loss, usually manifesting by 4 years of age.1
ANSD has a heterogeneous aetiology that includes both acquired and congenital factors. About 40% of cases have genetic origins, and inheritance patterns include mitochondrial, X-linked, autosomal dominant, and autosomal recessive inheritance.2 Otoferlin-encoding OTOF gene, PJVK, and GJB2 have been repeatedly implicated in ANSD, underscoring its genetic heterogeneity.3
Mostly diagnosed in the neonatal period, ANSD has a preference for neonates with perinatal risk factors such as prematurity, low birth weight, hyperbilirubinemia, hypoxia, infections, and neonates needing NICU intervention.4 This pathophysiological discordance creates important barriers to speech discrimination, especially in the presence of background noise, even when audiometric thresholds can be normal or even profoundly impaired.5
The diagnosis of ANSD is challenging because of its heterogeneous aetiologies and audiometric variability. Need for comprehensive testing: A minimum audiological test battery is required, including otoacoustic emissions (OAEs), cochlear microphonics (CMs), auditory brainstem response (ABRs), behavioural responses, middle ear function, and speech perception tests in quiet and noise.6 Treating ANSD is equally challenging due to the neural dys-synchrony that underlies the disorder, rendering many conventional interventions ineffective. Cochlear implants (CIs) offer a potential solution, but outcomes are good only in presynaptic etiologies.7
Literature on ANSD in twins is sparse. So this case report adds to the limited body of evidence, potentially sparking further research to deepen understanding of the epidemiology of its possible genetic basis and for screening ANSD in twins.
CASE REPORT
Monozygotic male twins Born in 2004 to a G4P5 mother from a non-consanguineous marriage via normal vaginal delivery at home, assisted by a midwife, presented to our Outpatient department with progressive bilateral hearing loss over 15 years. The twins experienced significant challenges understanding speech, particularly in noisy school environments, despite perceiving sounds, which adversely impacted their academic performance. They reported no other ear-related symptoms, such as discharge or pain. Their medical history showed no psychological or developmental issues, no NICU admissions, and no pre- or postnatal complications.
On examination of the pinna, the external auditory canal was normal with a normal intact tympanic membrane and normal vestibular function. No focal neurological deficit was observed.
Investigation
Twin - A
Pure tone audiometry revealed bilateral substantial hearing impairments. The right ear exhibited severe sensorineural loss, while the left ear exhibited moderate sensorineural hearing loss [Figure 1]. Speech audiometry further elucidated the extent of auditory dysfunction. The Speech Reception Threshold was 70 decibels Hearing Level (db HL) for the left ear and 100 dB HL for the right ear. The speech discrimination scores were significantly impaired, with the left ear performing only 5% and the right ear not being able to identify or repeat any words given (0% score). Tympanometry produced Type A graphs bilaterally, with the absence of stapedial reflexes. OAE testing, including screening and diagnostic procedures, yielded a bilateral pass, indicating preserved outer hair cell function within the cochlea [Figures 2a and b]. Brainstem Auditory Evoked Response (BERA) suggested the bilateral Vth peak was absent in both ears at 105 dB HL [Figures 2c and d]. Magnetic Resonance Imaging (MRI) of the inner ear and brain did not show any structural abnormality.
![Photograph of pure tone audiometry showing severe sensorineural hearing loss in right ear [red arrow] and moderate sensorineural hearing loss in the right ear [blue arrow].](/content/181/2025/6/1/img/AONO-6-e011-g001.png)
- Photograph of pure tone audiometry showing severe sensorineural hearing loss in right ear [red arrow] and moderate sensorineural hearing loss in the right ear [blue arrow].
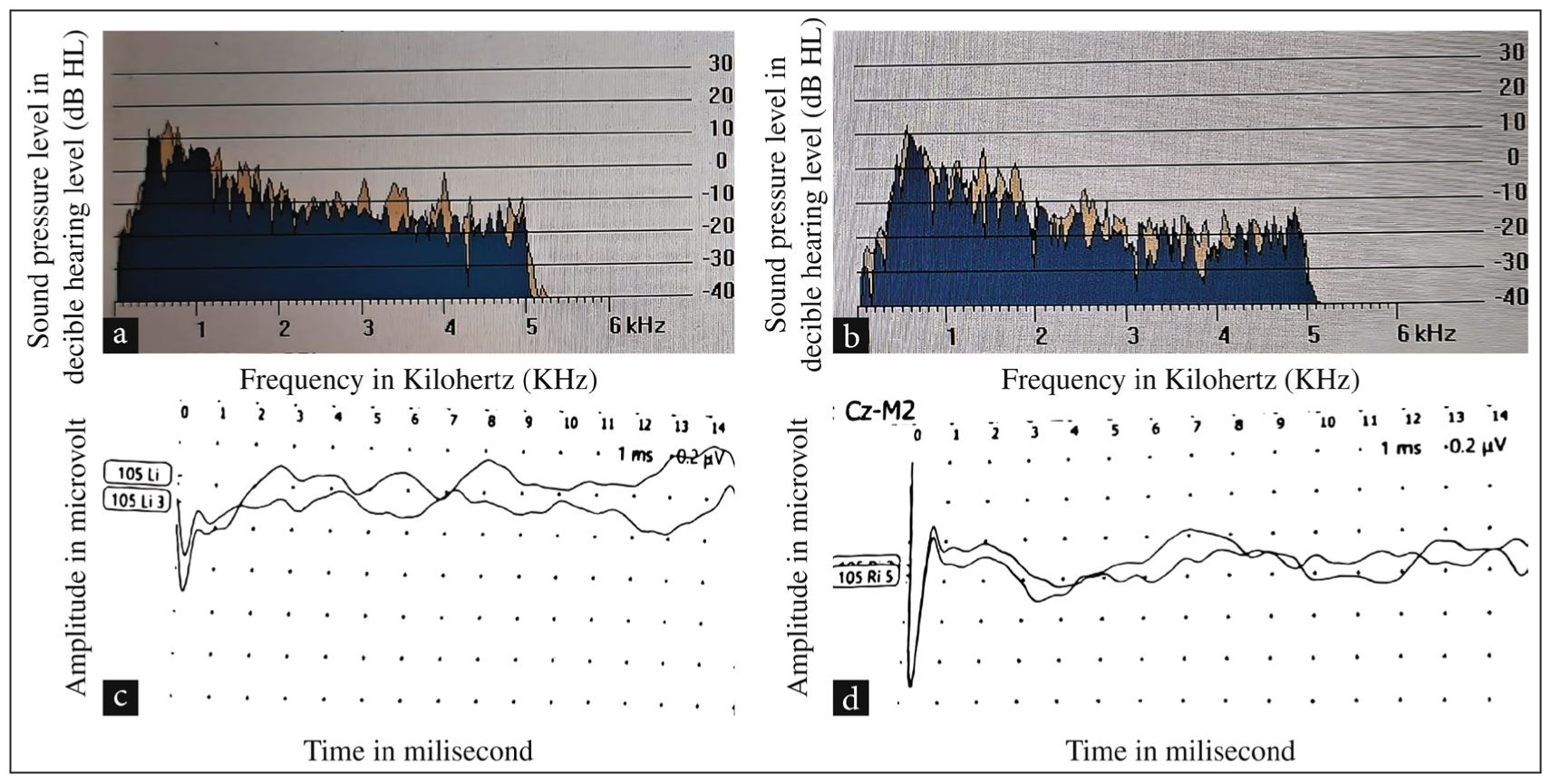
- (a) Photograph of transient evoked OAEs was present in the right ear of Twin A. (b) Photograph of transient evoked OAEs in the left ear of Twin A. (c) Photograph of Brainstem Evoked Response Audiometry showing Vth peak absent at 105 dB HL in the right ear of Twin A. (d) Photograph of Brainstem Evoked Response Audiometry showing Vth peak absent at 105 dB HL in the left ear of Twin A. OAE: Otoacoustic emissions, db HL: Decibel hearing level.
Twin - B
Pure tone audiometry of Twin B had inconsistent responses. Tympanometry yielded bilateral Type A graphs, indicating normal middle ear function, with the absence of stapedial reflexes bilaterally. OAE testing resulted in a bilateral pass [Figures 3a and b]. In contrast, the BERA bilateral Vth peak was absent in both ears at 105 dB HL [Figures 3c and d], with a normal MRI brain with an inner ear.
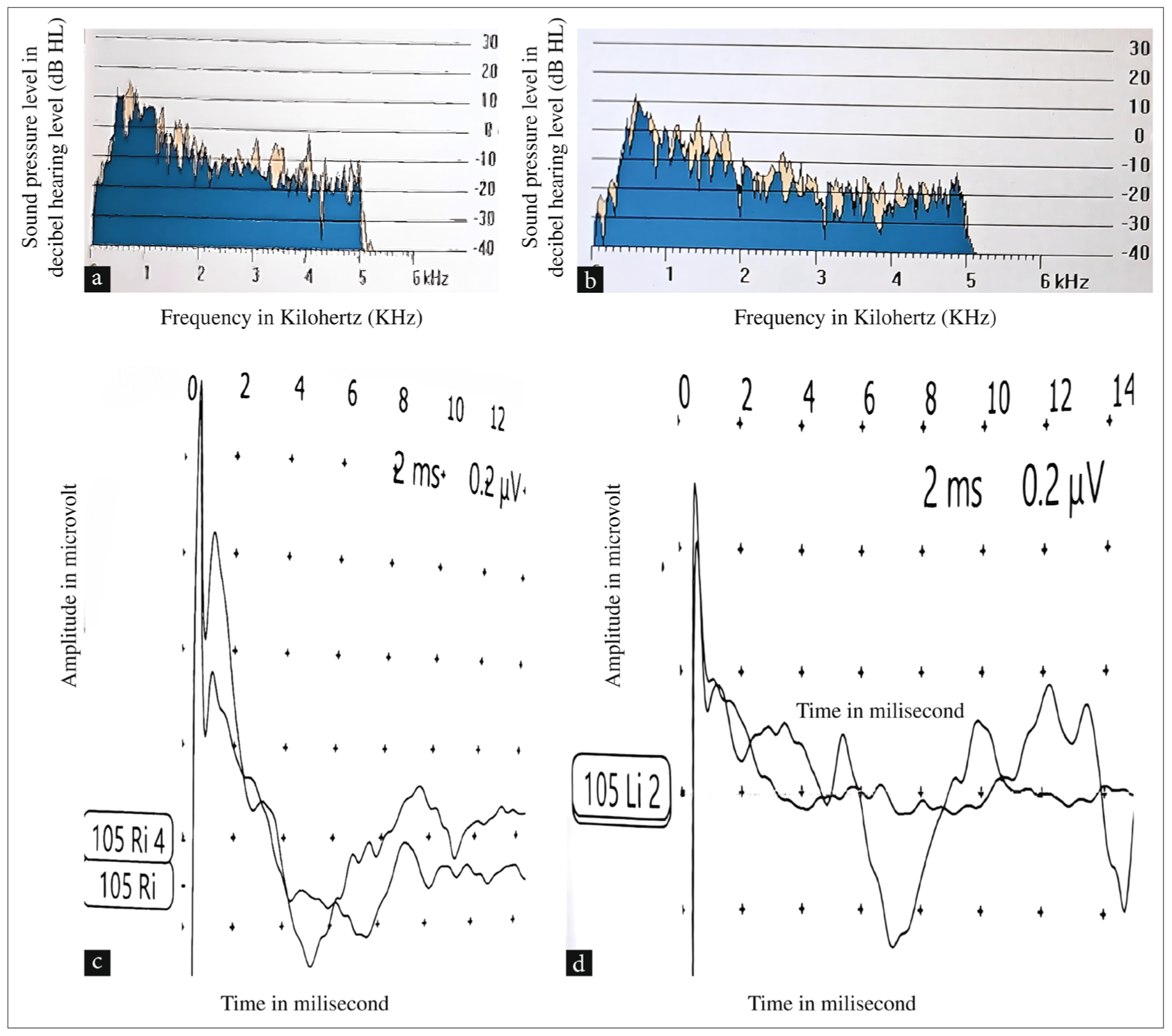
- (a) Photograph of transient evoked OAEs was present in the right ear of Twin B. (b) Photograph of transient evoked OAEs in the left ear of Twin B. (c) Photograph of Brainstem Evoked Response Audiometry showing Vth peak absent at 105 dB HL in the right ear of Twin B. (d) Photograph of Brainstem Evoked Response Audiometry showing Vth peak absent at 105 dB HL in the left ear of Twin B. OAE: Otoacoustic emissions, db HL: Decibel hearing level.
Management
Bilateral digital hearing aids were trialed for 3 months, with real-ear fits customized based on the twins’ audiometric characteristics. The aids were configured to emphasize speech frequencies, and follow-ups every 2 weeks involved subjective rating, speech perception tests in quiet and noise, and aided audiometry. No meaningful enhancements in speech discrimination or functional communication were noted, making amplification not useful.Single-sided cochlear implantation of the right ear in both twins, pending approval and scheduling.
Psychosocial support involves biweekly counselling sessions for the twins and family to manage emotional and social issues, including frustration due to communication problems and academic difficulties. Coping skills and peer support group involvement were facilitated by a clinical psychologist with expertise in hearing loss.
Family education sessions were conducted to facilitate understanding of ANSD, expectations of CIs, and long-term care needs.
DISCUSSION
ANSD presents as an interruption of auditory neural synchrony despite intact cochlear outer hair cell function, such as seen in the 19-year-old twins in this case. Their audiological profile of bilateral sensory neural hearing loss with bilateral Vth peak absent in both ears at 105 dB HL, normal OAE, and profoundly impaired speech discrimination (5% and 0%) is diagnostic of ANSD. The infrequency of ANSD in twins, combined with the lack of perinatal risk factors like NICU admission or prematurity, is suggestive of a potential genetic cause.8
The genetic underpinnings of ANSD are well established, with around 40% of instances being associated with mutations in genes like OTOF, PJVK, and GJB2. Shared genetic mutations may explain the concordant ANSD presentation in twins, particularly if monozygotic.2
Perinatal risk factors such as prematurity, hypoxia, and hyperbilirubinemia are highly linked with ANSD, especially in NICU populations where ANSD is responsible for 10%–15% of sensorineural hearing loss. While these twins did not have any reported perinatal complications, their common ANSD diagnosis implies potential subclinical environmental or genetic factors. Neonatal screening protocols relying solely on OAE (which will be normal in ANSD) BERA is usually done only if OAE fails. Incorporating BERA in routine screening, especially for inherently high-risk twins, would have facilitated earlier diagnosis.9
Management of ANSD in twins is complex due to the neural dyssynchrony that limits the efficacy of conventional hearing aids, as observed in the twins’ unsuccessful amplification trials. Cochlear implantation, planned for these patients, may offer potential benefits, particularly for presynaptic ANSD aetiologies, by bypassing defective neural pathways. However, outcomes vary, necessitating careful candidate selection and postoperative rehabilitation. Early diagnosis through neonatal OAE and BERA screening could have allowed interventions like speech therapy or cochlear implant candidacy to be initiated during critical developmental periods.6
The late diagnosis in this instance is due to the lack of such screening, which led to the twins’ significant speech and educational problems. Genetic screening is indicated to explore possible hereditary causes, considering the concordant presentation. Longitudinal follow-up is critical to track outcomes after cochlear implantation and measure developmental gains.
CONCLUSION
This case serves as a compelling call to action for improving neonatal screening methods by compulsory dual OAE and BERA (even if the child passes OAE), especially for the high-risk population. Managing ANSD poses great challenges based on the relatively low effectiveness of traditional treatments. Conventional hearing offers only limited benefits, leaving cochlear implantation as the option. However, CIs work efficiently when inserted in the earliest stage of a child’s life, emphasizing the importance of early diagnosis. Achieving a successful outcome requires striking a balance between treatment and psychosocial care. Improved screening, future research on the genetics of ANSD, and the results of CIs are crucial for better care.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
REFERENCES
- National Organization for Rare Disorders (NORD) 2021 [Last accessed 16 April 2025]. Available from: https://rarediseases.org/rare-diseases/auditory-neuropathy-spectrum-disorder/
- [Google Scholar]
- The Genetic Basis of Auditory Neuropathy Spectrum Disorder (ANSD). Int J Pediatr Otorhinolaryngol. 2011;75:151-8.
- [CrossRef] [PubMed] [Google Scholar]
- Genetic Etiological Analysis of Auditory Neuropathy Spectrum Disorder by Next-Generation Sequencing. Front Neurol. 2022;13:1026695.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- KidsHealth. 2021 [Last accessed 16 April 2025]. Available from: https://kidshealth.org/en/parents/ansd.html
- [Google Scholar]
- Speech Perception in Children with Auditory Neuropathy/Dyssynchrony Managed with Either Hearing Aids or Cochlear Implants. Otol Neurotol. 2008;29:179-82.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of Cochlear Implantation in Children with Auditory Neuropathy. Otol Neurotol. 2003;24:579-88.
- [Google Scholar]
- Auditory Neuropathy Spectrum Disorder: Its Prevalence and Audiological Characteristics in a Pediatric Population. Int J Audiol. 2015;54:519-26.
- [Google Scholar]
- Multi-Site Diagnosis and Management of 260 Patients with Auditory Neuropathy/Dys-synchrony (Auditory Neuropathy Spectrum Disorder). Int J Audiol. 2010;49:30-43.
- [CrossRef] [PubMed] [Google Scholar]








