Translate this page into:
Correlation between Carotid–Cochlear and Sensorineural Hearing Loss: A Cross-Sectional Study
Address for correspondence Arun Alexander, MS, DNB, Department of ENT, Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry 605006, India (e-mail: arunalexandercmc@gmail.com).
Abstract
Background
Sensorineural hearing loss is a condition with several etiologies and varies with the age of the individual. Carotid-cochlear interval is the minimum distance between basal turn of cochlea and the genu of petrous part of internal carotid artery. It is believed that constant pulsations from carotid can cause fluid pressure changes within the cochlea leading to damage to hair cells causing hearing loss.
Objective
To study the correlation between carotid-cochlear interval and degree of hearing loss at different frequencies in patients with sensorineural deafness and compare this interval with normal subjects.
Methods
Seventy cases with sensorineural hearing loss between 18 and 60 years undergoing HRCT temporal bone were grouped together and 70 cases with normal hearing undergoing CT nose and paranasal sinuses were grouped together. Carotid–cochlear interval measured in both the groups was correlated with the degree and frequency of hearing loss and compared with normal subjects.
Results
The mean carotid–cochlear interval in sensorineural hearing loss and in normal subjects was found to be 1.30 + 0.68 (SD) mm and 1.83 + 0.74 (SD) mm, respectively with p < 0.001. The coefficient of correlation between carotid-cochlear interval and pure tone average in patients with sensorineural deafness was r = –0.740 with p-value < 0.001.
Conclusion
Carotid-cochlear interval is significantly low in patients with sensorineural hearing loss and bears a strong negative correlation with the degree of hearing loss at mid- and high-frequency ranges. Thus we hypothesize that pulsations from carotid artery cause damage to hair cells in the organ of Corti producing audiological symptoms such as hearing loss.
Keywords
carotid–cochlear interval
cochlear implantation
sensorineural hearing loss
Introduction
Sensorineural hearing loss (SNHL) is a multifaceted condition with profound medical, social, and cultural ramifications. There are several congenital and acquired, genetic, and nongenetic (syndromic and nonsyndromic) causes of SNHL. Its etiology varies with the age of the individual affected. It is believed that chronic stimulation of the cochlea by loud sounds either originating from the outside or from the body itself can damage the cochlea leading to hearing loss. It is believed that the close relation of the cochlea to the carotid artery in some individuals may lead to hearing loss in some frequencies.12 Reasons for why a person gradually goes deaf are at times difficult to assess, and this study endeavors to explore one such probable cause. Cochlear implantation surgery is one of the modalities to treat deafness, which has revolutionized the treatment and prognosis of profound SNHL.3 The carotid–cochlear interval (CCI) is an important landmark that has to be assessed prior to implantation due to their close relationship within petrous temporal bone and the risk of inadvertent implantation into the carotid artery if the interval is membranous.1
Thus this study aims at finding the normal variation of CCI in the sample of Indian population, its implications on the etiology of hearing loss, and its importance as a preoperative assessment prior to cochlear implantation.
Methods
Patient population included patients seen in the ENT (ear-nose-throat) clinic during the study period of September 2016 to October 2017. Two groups with 70 cases in each were enrolled in this study. Institute ethics committee approval was obtained before starting the study. Informed consent was obtained from patients. All diagnosed patients of SNHL between 18 and 60 years of age were seen in the ENT clinic, and all patients undergoing computed tomographic (CT) scan of the nose and paranasal sinuses with normal hearing during the same period were included in the study. Patients with middle ear disease, structural lesions of the temporal bone, trauma to the temporal bone, and neurologic causes affecting the eighth cranial nerve were excluded from the study.
Two Groups
Two groups were studied:
Group 1: SNHL patients between 18 and 60 years of age, who met the aforementioned inclusion and exclusion criteria.
Group 2: Patients undergoing CT scan of the nose and paranasal sinuses with normal hearing seen during the study period.
Variables analyzed include independent variables such as age, sex, and side of the ear, and outcome variables such as CCI in mm, degree of hearing loss at different frequencies in dB, and pure tone average (PTA) in dB.
Statistical Analysis
Continuous variables such as CCI, age, and frequency of hearing loss were expressed as mean and standard deviation (SD).
The correlation between CCI and degree of hearing loss at various frequencies was assessed by Pearson correlation as data followed a normal distribution (►Fig. 1).
The CCI across the groups classified based on the frequencies was analyzed by one-way analysis of variance (ANOVA) as data followed a normal distribution.
The CCI was compared across groups 1 and 2 with independent t-test as data followed normal distribution.
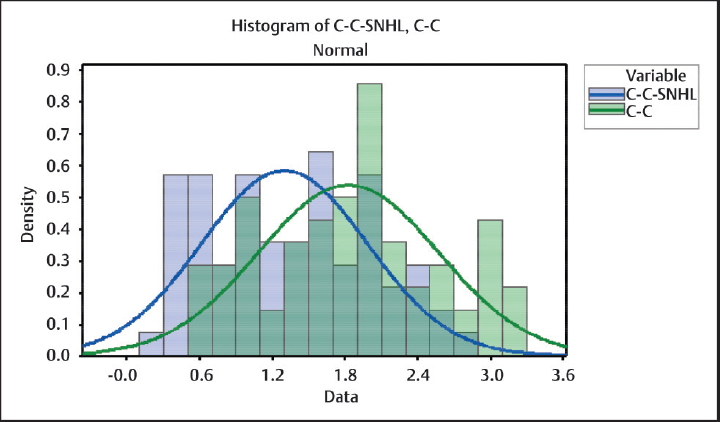
- Graph showing the distribution of CCI in both the groups. CCI, carotid-cochlear interval.
Results
In this study, the age group of patients with hearing loss varied between 18 and 56 years with a mean of 36 years and among normal patients between 19 and 50 years with a mean of 33.1 years, with no statistical difference between them (p = 0.07). Sex distribution showed 46 (65.7%) males and 24 (34.3%) females in group 1 and 40 (57.1%) males and 30 (42.9%) females in group 2, with no statistical significance between them (p = 0.298). Side of the ear studied showed 34 (48.6%) right and 36 (51.4%) left in group 1 and 35 (50%) each on right and left side in group 2.
Correlation between CCI and age in SNHL patients showed r = −0.075, p –0.539 (not significant). Comparison between CCI and sex of the individual studied showed p = 0.34 (not significant), and similarly comparison with the side of the ear studied showed p = 0.70 (not significant).
Comparison between CCI among the two groups showed p < 0.001 which is statistically significant (►Table 1). Correlation between CCI and PTA showed r = –0.740, p < 0.001 (statistically significant) (►Table 2). Similarly, correlation between CCI and groups divided based on frequency (►Fig. 2) as low (250 Hz), mid/PTA (500 Hz,1 kHz, and 2 kHz), and high (4 and 8 kHz) showed r values of –0.634, –0.740, and –0.706, respectively, with p < 0.001 for all of them (►Table 3). Comparison of CCI between these groups showed p = 0.03 (statistically significant). Patients with SNHL were divided based on the degree of hearing loss as mild, moderate, severe, very severe, and profound hearing loss, and comparison of CCI between them showed a statistically significant difference between the mean with p < 0.001 (►Fig. 3).
| Groups | n | Min (mm) | Mean (mm) | Max (mm) | SD (mm) |
|---|---|---|---|---|---|
| Group 1 | 70 | 0.2 | 1.30 | 2.8 | 0.68 |
| Group 2 | 70 | 0.5 | 1.83 | 3.3 | 0.74 |
Abbreviations: CCI, carotid–cochlear interval; SD, standard deviation.
p < 0.001 (significant).
Statistical test used: unpaired t-test.
| Variables | Minimum | Mean | Maximum | SD |
|---|---|---|---|---|
| PTA (dB) | 28.3 | 58.42 | 106.6 | 20.39 |
| CCI (mm) | 0.2 | 1.30 | 2.8 | 0.68 |
Abbreviations: CCI, carotid–cochlear interval; PTA, pure tone average; SD, standard deviation; SNHL, sensorineural hearing loss.
Coefficient of correlation= −0.740, p < 0.001 (significant).
Statistical test: Pearson correlation.
| Frequency | n (70) | Min (mm) | Mean (mm) | Max (mm) | SD (mm) |
|---|---|---|---|---|---|
| Low | 4 | 2 | 2.15 | 2.6 | 0.30 |
| Mid | 15 | 0.5 | 1.23 | 2.4 | 0.59 |
| High | 51 | 0.2 | 1.25 | 2.8 | 0.69 |
Abbreviations: CCI, carotid–cochlear interval; SD, standard deviation.
p = 0.03 (significant).
Statistical test used: one-way ANOVA.
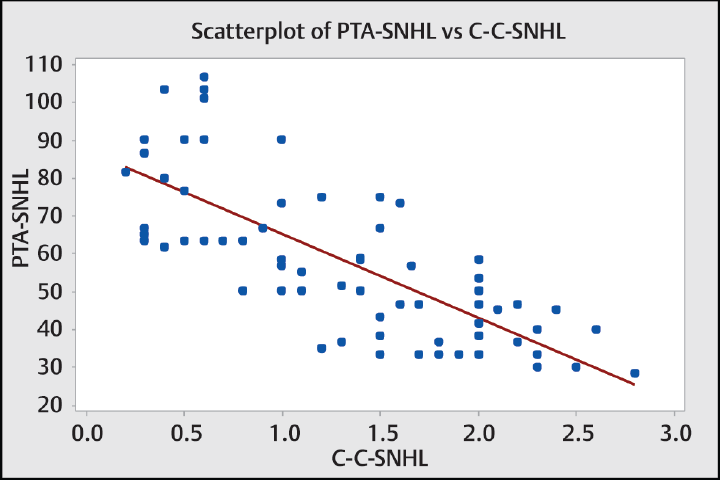
- Graph showing the relationship between CCI (mm) and pure tone average (dB) in patients with SNHL. SNHL, sensorineural hearing loss.
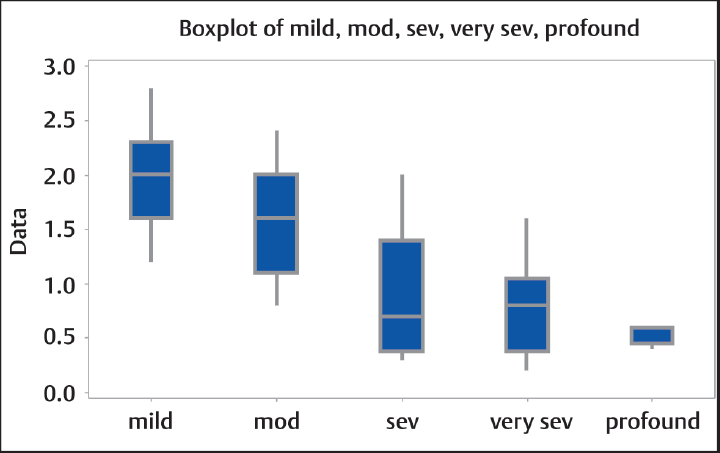
- Graph of CCI among groups according to the degree of hearing loss. The difference between groups is significant (p < 0.001). CCI, carotid-cochlear interval; mod, moderate; sev, severe.
Discussion
CCI extends from the basal turn of the cochlea to the genu of the vertical and horizontal portions of the petrous part of the internal carotid artery. The petrous portion of the temporal bone contains various vascular, neuronal, and labyrinthine structures in a small area (►Fig. 4) and (►Fig. 5).2 The vertical portion of petrous part of internal carotid artery enters the carotid canal lying in the petrous temporal bone and takes a turn anterior and medial and runs horizontally. In this study, this relationship between the cochlea and artery and its implications have been critically analyzed in both normal individuals and patients having hearing loss.

- High Resolution CT showing genu of carotid artery and basal turn of cochlea. CT, computed tomography.

- Right side carotid-cochlear interval (enlarged view).
Among the group with SNHL, 62.8% of the patients had bilateral pathology at the time of presentation. Majority of the patients belonged to the age group of 31 to 40 years (55.5%). The condition was more common in males than in females, with 65.7% being males.
The CCI range in normal individuals was 0.5 to 3.3 mm (mean –1.83 ± 0.74) and in SNHL patients it was 0.2 to 2.8 mm (mean –1.30 ± 0.68). There have been reports of total absence of the bone leading to a dehiscence between the carotid artery and the basal turn of the cochlea. However, in most studies this range lies between 0.2 and 6.2 mm.2,4 The widest distance has been reported to be 9 mm.5 This variation is likely due to the influence of factors that determine the space available for pneumatization between the different structures of the petrous pyramid of the temporal bone.4
In patients with SNHL’ the correlation between CCI and age was found to be r = –0.075 and p = 0.33, which did not exhibit any statistical significance. In males’ CCI was found to range between 0.2 and 2.8 mm and in females 0.3 and 2.0 mm with no significant relationship between them (p = 0.34). The range on the right side was found to be between 0.3 and 2.5 mm and on left side 0.2 and 2.8 mm, with no statistically significant relationship (p = 0.70). Similar findings were noted in normal patients with no significant relationship between these variables. Thus age, sex, and side of the ear did not have any influence on the normal variation in CCI, which is similar to the findings of other studies.2,4-6 This implies that this portion of the petrous temporal bone is completely developed at the time of birth with no change occurring over time.
Carotid-Cochlear Interval and Degree of Hearing Loss
The correlation between PTA of patients with SNHL and CCI in this study was found to be r = –0.74 with p < 0.001 (►Fig. 4), which is statistically significant with negative correlation implying that as CCI decreases, degree of hearing loss increases. These findings were unlike those by Cetin et al5 who reported longer CCI in patients with mid tone SNHL. We believe that this may be due to population differences or difference in the methods used in this study.
Young et al2 and Modugno et al7 reported cases with dehiscence of the bone between the cochlea and internal carotid artery presenting with high and mid tone SNHL. Gunbey et al8 found a similar finding when they tried to correlate hearing loss with CCI in patients presenting with tinnitus. Thus, the hypothesis proposed by Young et al2, which states that pulsations from the internal carotid artery might create fluid pressure changes and direct stimulation of hair cells on the basilar membrane, is strongly upheld by this study.
Carotid-Cochlear Interval in Normal Patients and Patients with Sensorineural Hearing Loss
There lies a statistically significant difference between the CCI of normal and patients with SNHL and also between the groups divided based on the degree of hearing loss. This possibly signifies the importance of CCI in the etiology of SNHL and the need to measure this distance in such patients in whom all other possible, common etiologies have been ruled out. In the case reported by Young et al,2 the patient with dehiscent CCI presented with fluctuating SNHL. Though no case with bony dehiscence was detected in this study, the shortest CCI measured was 0.2 mm, where only the bone of the otic capsule separates the cochlea from the carotid artery. This can transmit high-pressure pulsations of the carotid to the cochlea and cause damage to the hair cells.
Carotid-Cochlear Interval and Frequency of Hearing Loss
The correlation between CCI and frequency of hearing loss divided into low (250 Hz), mid (500, 1, and 2 kHz), and high (4 and 8 kHz) frequency showed a statistically significant strong negative correlation in mid (r = –0.734) and high (r = –0.706) frequencies. These findings reinforce the fact that internal carotid artery affects the hair cells situated in the basal turn of cochlea when compared with apical turns. As the diameter of cochlear canal decreases from base to apex, the diameter of the basilar membrane increases. Thus, maximal high frequency (20 kHz) resonates the base whereas maximal low frequency resonates the apical turns (20 Hz). Most of the basal turn is responsible for mid tone frequencies between 1 and 5 kHz. These findings reassure the findings in the two case reports that had dehiscent CCI and mid-high SNHL.
Thus, on analyzing the above variables, we propose that when a patient presents with unilateral or bilateral SNHL in mid and high frequency in the age group of 31 to 40 years, the possibility of reduced CCI being the etiology should be kept in mind.
The probable mechanism by which hearing loss occurs may be similar to that of noise and vibration-induced hearing loss9,10 wherein there may be a decrease in cochlear blood flow or a decrease in oxygen level within the cochlea leading to ischemia of hair cells, subsequently leading to apoptosis or necrosis of hair cells. Furthermore, the role of reactive oxygen species that occurs due to imbalance between oxygen supply and demand within the cochlea can also lead to damage to the hair cells. This opens more avenues for further research into the pathophysiology behind this condition, its diagnosis, and management. The idea of interrupting this interaction between the carotid artery and cochlea by surgical intervention, though highly risky, can be a possibility in the future.
Implications of the Study on Cochlear Implantation
Cochlear implantation is a method of auditory rehabilitation of patients with severe to profound hearing loss. It has become a relatively routine surgical procedure with great benefits in terms of communication enhancement and quality of life with minimal risks with both minor and major complications involved. It is performed in a relatively small area of petrous pyramid with close relationship between end organs of hearing and balance and the brain, facial nerve, and major blood vessels such as the internal carotid artery.
There have been three reports of misplaced cochlear implants into the carotid artery,11-13 which could have been avoided by understanding the close relationship between the cochlea and carotid artery, normal range of variation in this distance, and preoperative assessment of this distance using high-resolution CT temporal bone as proposed by Nevoux et al12. This might help the surgeon to be more vigilant whenever this distance is very short while making a cochleostomy and inserting the electrode array.
Conclusion
This study evaluated the CCI in normal patients and patients with SNHL and found a statistically significant relationship between the two groups. The CCI was found to have a strong negative correlation with degree of hearing loss, and this predominantly affects the mid- and high-frequency spectra. Thus, this study supports the hypothesis that pulsations of the internal carotid artery affect the hair cells in the basal turn of the cochlea causing damage to them and leading to SNHL.
Conflict of Interest
None.
References
- MDCT assessment of the cochlear-carotid interval. Neuroradiol J. 2011;24(3):439-443.
- [CrossRef] [PubMed] [Google Scholar]
- The cochlearcarotid interval: anatomic variation and potential clinical implications. Am J Neuroradiol. 2006;27(7):1486-1490.
- [Google Scholar]
- Cochlear implantation for the treatment of deafness. Annu Rev Med. 2004;55(1):157-167.
- [CrossRef] [PubMed] [Google Scholar]
- The cochlea and the carotid canal. Acta Radiol. 1990;31(1):33-35.
- [CrossRef] [PubMed] [Google Scholar]
- The importance of carotid-cochlear interval in the etiology of hearing loss. Indian J Otolaryngol Head Neck Surg. 2013;65(4):345-349.
- [CrossRef] [PubMed] [Google Scholar]
- The cochlear-carotid interval: preoperative assessment for cochlear implant. Mediterr J Otol. 2008;4:19-23.
- [Google Scholar]
- Bilateral dehiscence of the bony cochlear basal turn. Arch Otolaryngol Head Neck Surg. 2004;130(12):1427-1429.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of the cochlear-carotid interval on tinnitus perception. Surg Radiol Anat. 2016;38(5):551-556.
- [CrossRef] [PubMed] [Google Scholar]
- Sound-induced cochlear ischemia/hypoxia as a mechanism of hearing loss. Noise Health. 1999;2(5):17-32.
- [Google Scholar]
- Risk of hearing loss among workers with vibration-induced white fingers. Am J Ind Med. 2014;57(12):1311-1318.
- [CrossRef] [PubMed] [Google Scholar]
- The potential risk of carotid injury in cochlear implant surgery. Laryngoscope. 2002;112(2):262-266.
- [CrossRef] [PubMed] [Google Scholar]
- Cochlear implant in the carotid canal. Case report and literature review. Int J Pediatr Otorhinolaryngol. 2010;74(6):701-703.
- [CrossRef] [PubMed] [Google Scholar]
- Cochlear implant electrode misplaced in the carotid canal. Arch Otolaryngol Head Neck Surg. 2007;133(8):827-829.
- [CrossRef] [PubMed] [Google Scholar]







