Translate this page into:
The Distensibility of Reissner's Membrane: A Comparative Analysis
Address for correspondence Daniel J. Pender, MSE MD FACS, 145 West 86th Street #1C, New York City, NY 10024, United States (e-mail: djp2@cumc.columbia.edu).
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
Distention of Reissner's membrane in endolymphatic hydrops is a classical otopathologic finding in cases of Meniere's disease. A recent double hit analysis raised the possibility that the variability in the distensile behavior in Reissner's membrane may contribute to the vagaries of membrane pathology encountered in this disease. Such distensile variability is suspected to stem from the viscoelastoplastic behavior of the type IV collagen in Reissner's basement membrane.
Objective
To analyze the known distensile characteristics of Reissner's membrane for evidence of viscoelastoplastic behavior.
Methods
Extant data on human Reissner's membrane were analyzed for distensile characteristics. These features were then compared to the known characteristics of viscoelastoplasticity as manifested by polymers in general as well as a variety of collagenous tissues. These tissues included a synthetic collagen membrane as well as selected mammalian tissues.
Results
The limited extant data on human Reissner's membrane distensile behavior was found to manifest sigmoid load deformation at a lower strain rate of 0.47%/sec and a rigid rupture pattern at a 10-fold higher strain rate of 5.5%/sec. These characteristics were found to be similar to the general characteristics of polymer viscoelasticity, namely a sigmoid load deformation pattern at lower strain rate that stiffens and straightens as strain rate increases. Tensometric data from a synthetic collagen membrane and selected mammalian tissues were found to exhibit comparable load deformation patterns. These findings support the conclusion that human Reissner's membrane behaves in a viscoelastoplastic manner.
Conclusions
Human Reissner's membrane appears to exhibit viscoelastoplastic behavior comparable to that observed in other collagenous tissues. Such variable distensile behavior provides insight into why the degree of lesion distention before rupture in Meniere's disease might vary depending on the dynamics of membrane loading and the resultant rate of membrane strain.
Keywords
balance diseases
hearing loss
inner ear conditions
Meniere's disease
otology
Introduction
Distention of Reissner's membrane in endolymphatic hydrops is a classical otopathologic finding in cases of Meniere's disease.1 However, the biomechanics of human Reissner's membrane have been described as ‘quite puzzling.’2 A recent double-hit analysis raised the possibility that variability in the distensile behavior in Reissner's membrane may contribute to the vagaries of membrane pathology encountered in this disease.3 Such distensile variability may be attributable to viscoelastoplastic behavior of collagen in Reissner's membrane as suggested in a recent model.4
Such tissue viscoelastoplastic distensile behavior can be quantitatively assessed in vitro using a tensometer where load, deformation degree, and rate can be precisely con- trolled.5 Despite this, these parameters are not well-defined at present due to Reissner's membrane's diminutive size and delicacy, and the difficulty attendant to obtaining fresh human postmortem tissue for tensometric analysis. The objective of this study is to determine if the scant load deformation data that have been reported for Reissner's membrane is consistent with viscoelastoplastic behavior as demonstrated by other tissues.
Methods
Extant load deformation data on human Reissner's membrane were extracted from studies that utilized fresh post mortem tissue.6,7 In these studies, such tissue was subject to ex-vivo tensometric analysis in a device designed to strain the tissue specimen in a controlled manner to quantify its distensile behavior.
Tensometric data on human Reissner's membrane were compared to the general viscoelastoplastic behavioral characteristic of polymers for evidence of similarity.8 Such comparison focused on the characteristic of the stress-strain relationship as well as sensitivity to strain rate. This examination of distensile behavior was then extended to several collagenous tissues to assess consistency. These tissues included as artificial membrane composed of precipitated collagen fibers with a mesh-like molecular configuration similar to that of the type IV collagen found in Reissner's basement membrane.5 They also included selected mammalian tissues, specifically porcine cornea,9 bovine cornea,10 and caprine skin11 to validate the existence of viscolelastoplastic behavior in other mammalian tissues.
Results
Reissner's Membrane
Two sources were identified that reported quantitative in-vitro data on the distensile behavior of human Reissner's membrane.6,7 Both used tensometric devices similar to that employed for evaluation of the synthetic collagen matrix.5 In the earlier study, Reissner's membrane was reported to rupture abruptly in a brittle manner when strained at the higher rate of 5.5% per second.6 In the later study, a sigmoid response was observed when Reissner's membrane was strained at the lower rate of 0.47% per second.7 These responses are presented schematically in ►Fig. 1, after Ishii6 and Tanaka.7

- Human Reissner's membrane extensibility at different strain rates.
General Polymers
Polymers generally exhibit a sigmoid response pattern at low strain rate consisting of an initial viscous region of very low but gradually increasing stiffness, followed by a linear region of constant stiffness, and finally a plastic region of decreasing stiffness as the specimen approaches rupture. Additionally, polymers exhibit a sensitivity to strain rate wherein the initial viscous region is progressively lost as the tissue displays an earlier onset of increasing stiffness as strain rate rises leading to rupture at a lower degree of strain.4 These features are shown in ►Fig. 2.
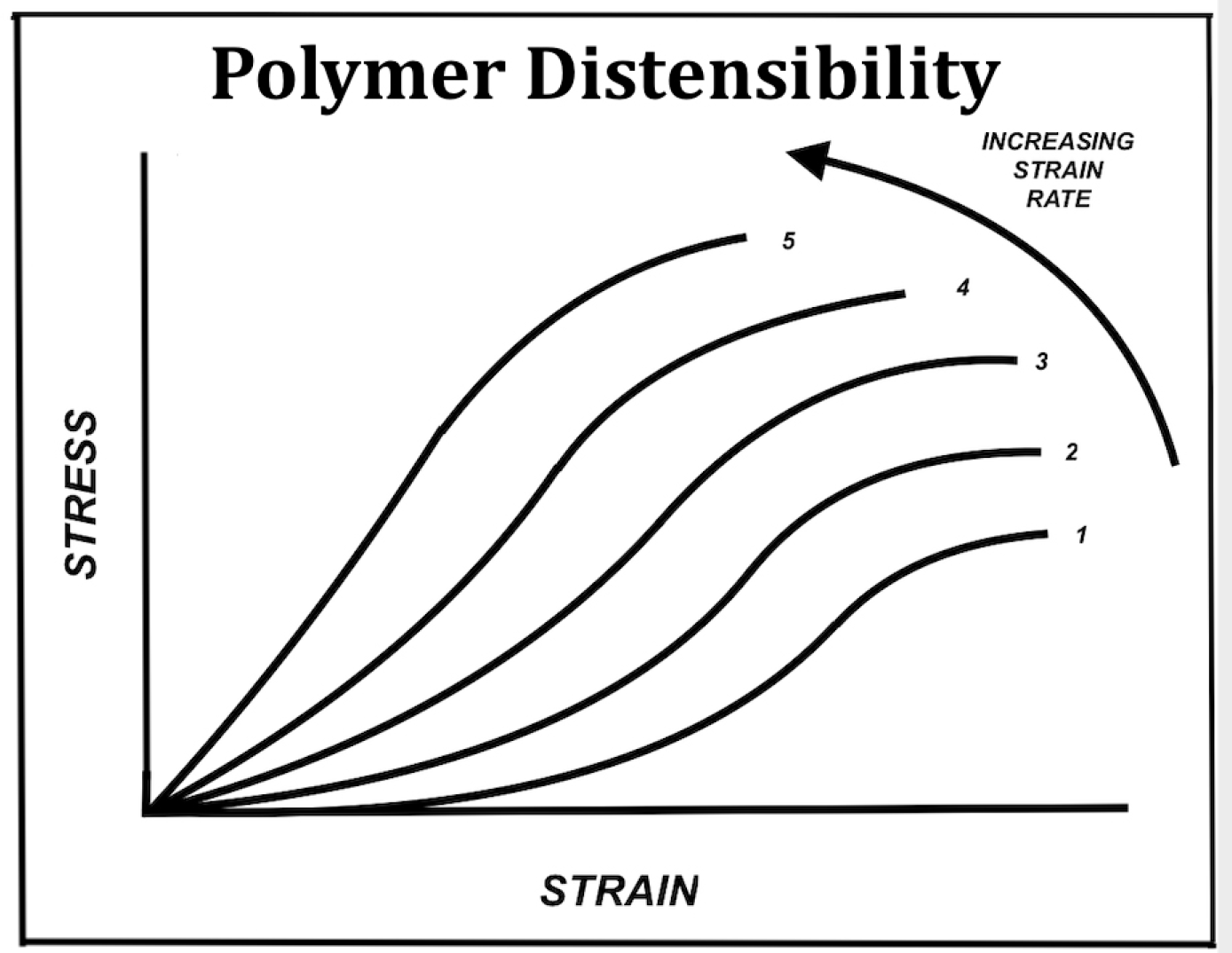
- Schematic of polymer distensibility and variation with strain rate
Collagen Matrix
Reported data on a synthetic collagen membrane were found to exhibit a sigmoid viscoelastoplastic load deformation pattern. This response consisted of an initial visco-elastic region of increasing stiffness, followed by a linear elasto-plastic region, and then a terminal plastic region of decreasing stiffness leading to rupture. This is illustrated in ►Fig. 3, after Roeder.5 These features were reported as strain rate-sensitive, becoming less exaggerated as the strain rate increased and leading to progressively earlier rupture. Such distensile features are consistent with those exhibited by general polymers described above.

- Load-deformation curve for a synthetic collagen matrix
Mammalian Tissues
Reported data on the deformation behavior of porcine cornea,9 bovine cornea,10 and caprine skin11 all demonstrated similar tensometric responses. All were found to exhibit similar sigmoid load deformation responses. The results for porcine cornea are shown in ►Fig. 4, after Su..9 This sigmoid pattern of stress versus strain mimics that exhibited by the synthetic collagen membrane noted above. Here the toe region is designated viscous zone and considered to represent the ‘normal physiologic’ operating range of the tissue. The adjacent linear elastic segment is considered ‘dangerous’ since it leads to the plastic zone with incipient rupture.

- Porcine cornea displays sigmoid distention pattern
A similar sigmoid distensile response is found for bovine cornea, as shown in ►Fig. 5, after Liu.10 Ruptures in both porcine and bovine specimens occur at approximately at the same level of stress in the range of 4 to 5 MegaPascals.
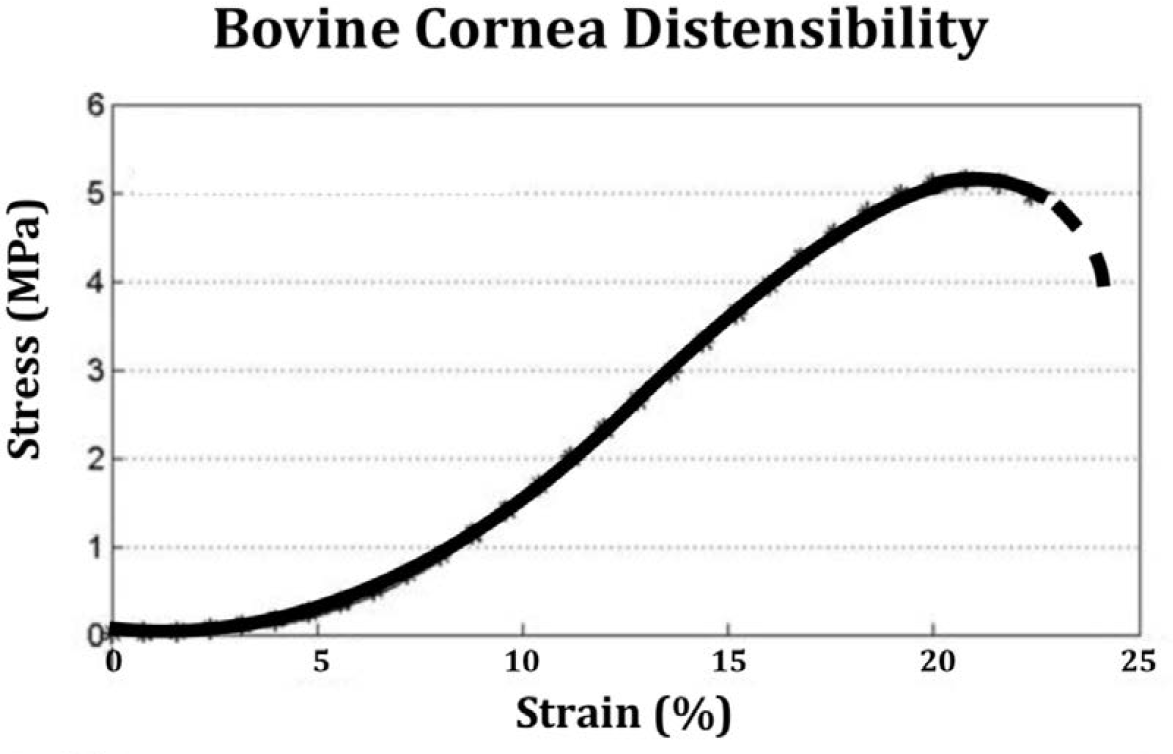
- Bovine cornea displays a sigmoid distention pattern
Reported data on caprine skin were found to exhibit a variable load deformation response to tensile testing as shown in ►Fig. 6, after Arumugam.11 At the lower strain rate of 5% per minute, this tissue demonstrated the typical sigmoid viscoelastoplastic pattern with substantial distention at low stress levels. However, at a higher strain rate of 1000% per minute, the initial viscous toe region was effectively lost, and the overall behavior of the specimen becomes rigid and quasi-linear.

- Caprine skin demonstrates a sigmoid distension pattern at the lower strain rate and a quasi-linear pattern at the higher strain rate
Discussion
Summary of Results
Reported tensometric data on human Reissner's membrane exhibit load deformation features that are consistent with tissue viscoelastoplasticity. A sigmoid load-deformation response is exhibited at the lower rate of 0.47% per second, while a rigid response has been reported at the higher strain rate of 5.5% per second. These behaviors are consistent with the viscoelastoplastic response characteristics displayed by polymers in general, by a synthetic collagen membrane and those reported for selected mammalian tissues. All tissues demonstrated a sigmoid response pattern consisting of an initial viscous ‘toe’ zone of increasing stiffness followed by an elastic ‘transition’ zone of constant stiffness followed by a plastic ‘heel’ zone of steadily decreasing stiffness leading to rupture.
Analysis of Results
The focus of the current study is on whether Reissner's membrane is viscoelastoplastic. Such viscoelastoplasticity is known to be a more complicated response to stress than that of simple elasticity. Simple elasticity involves a time invariant linear response of the material wherein energy is stored in the material and deformation is fully reversible. Simple elastic distention is near instantaneous and stress propagates through the material in approximately 10-12 seconds. It reflects interatomic bond deformation where stress and strain are proportional in accordance with Hook's law. It is typically seen in solid materials with a crystalline lattice structure.
In contrast, polymers are composed of long folded molecular chains. These molecules exhibit a more complex behavior. The prefix ‘visco’ implies that a fluid-like molecular movement may precede the purely elastic response. This viscous component is due to straightening of the polymer molecule, a flow process that is time dependent and associated with the release of energy as heat. This process is fully reversible and heat is reabsorbed. Distention of such materials is usually strain rate sensitive, meaning that it takes more force to stretch the material quickly due to the presence of the viscous element. If force is applied abruptly, the viscous element will may not have time to permit significant molecular rearrangement and if the applied force is sufficient, the elastic covalent bonds in the polymer backbone will rupture abruptly in a brittle manner with little distention.8
Finally, the suffix ‘plastic’ implies that a permanent distention component may succeed the viscous and elastic phases if the material is deformed gradually. This can be understood to mean that the molecular polymers are fully straightened, the elastic interatomic bonds within the polymer backbone are stretched maximally but not broken, and that the long straightened polymer molecules are sliding by each other due to random rupture of hydrogen bonding between adjacent polymer chains. This process is of course irreversible and thus the designated plastic.
In the case of human Reissner's membrane, this tentative confirmation of viscoelastoplastic behavior may help to explain the variation in pathological response in endolymphatic hydrops. Although certain hydropic lesions exhibit rupture with little distention, others exhibit pronounced distention without rupture along with an array of intermediate states.1 This would suggest that viscoelastoplastic strain rate sensitivity might be a controlling factor in lesion behavior in Meniere's disease.
Critical Analysis of Methods
One difficulty encountered in this study is that the amount of quantitative data on the distensile behavior of Reissner's membrane cited herein is extremely limited. However, there have been a number of qualitative observations on the viscoelastic behavior of Reissner's membrane that are of historical interest. Bekesy described experiments wherein he noted that the ‘elasticity of Reissner's membrane was high.’12 Tonndorf commented on the viscoelastic behavior of Reissner's membrane but did not present any quantitative data.13 Flock reported a qualitative observation of viscoelastic behavior wherein a ruptured Reissner's membrane was ‘initially wavy in appearance but gradually straightened.’14
A second difficulty is that the reported quantitative data are not reported in a uniform way. Most studies cited herein present membrane loading as stress in MegaPascals, while the testing on Reissner's membrane reported the loading in milliNewtons. Certain reports present distention in absolute terms, i.e., microns, while others report strain as a percentage. These differences in reporting parameters constitute an impediment to an exact comparison of data. However, the quantitative characteristics of the data that are available, reflecting a sigmoid distensile response at lower strain rates and a linear, brittle response at higher strain rates, are nevertheless consistent with a viscoelastoplastic process.
Finally, the scant extant data on human Reissner's membrane necessitated a comparison with other collagenous tissues.15 This analysis of material behavior is based on the assumption that the collagen tissues in general share mutual constitutive qualities that underlie their distensile behavior, while displaying variation in the degree of their distensile behavior.16 Conclusions deriving from such a comparison should therefore be taken as tentative. Nevertheless, the consistency of loading response of Reissner's membrane with those of other tissues appears compelling.
Conclusion
Human Reissner's membrane appears to exhibit distensile behavior consistent with viscoelastoplasticity as observed in other mammalian tissues. Such viscoelastoplastic disten- sibility may provide insight into why the degree of lesion distention before rupture in Meniere's disease might vary depending on the dynamics of membrane loading and the resultant membrane distensile response.
Conflict of Interest
None declared.
Funding
None.
References
- Pathology of Meniere's disease. Ann Otol Rhinol Laryngol Suppl. 1984;112(4)(suppl):31-35.
- [CrossRef] [PubMed] [Google Scholar]
- Stiffness of Reissner's membrane. J Acoust Soc Am. 1974;56(4):1252-1257.
- [CrossRef] [PubMed] [Google Scholar]
- The biomechanics of lesion formation in endolymphatic hydrops: single and double hit mechanisms. Otol Neurotol. 2019;40(3):398-403.
- [CrossRef] [PubMed] [Google Scholar]
- A model of viscoelastoplasticity in the cochleo-saccular membranes. Laryngoscope Investig Otolaryngol. 2019;4(6):659-662.
- [CrossRef] [PubMed] [Google Scholar]
- Tensile mechanical properties of three-dimensional type I collagen extracellular matrices with varied microstructure. J Biomech Eng. 2002;124(2):214-222.
- [CrossRef] [PubMed] [Google Scholar]
- The Physical Strength of the Membranous Labyrinth and Its Relation to Endolymphatic Hydrops. In: Kitahara M, ed. Meniere's Disease. Tokyo: Springer; 1990.
- [CrossRef] [Google Scholar]
- Measurement of pressure and displacement of the membranous labyrinth in endolymphatic hydrops by the tensile test. Acta Otolaryngol Suppl. 1997;528:30-36.
- [Google Scholar]
- Engineering Viscoelasticity. 2001. 2021. at: http://ocw.mit.edu/courses/materials-science-and-engineering/3-11-mechanics-of-materials-fall-1999/modules/visco.pdf (accessed )
- [Google Scholar]
- Corneal hyper-viscoelastic model: derivations, experiments, and simulations. Acta Bioeng Biomech. 2015;17(2):73-84.
- [Google Scholar]
- A mechanical model of the cornea considering the crimping morphology of collagen fibrils. Invest Ophthalmol Vis Sci. 2014;55(4):2739-2746.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of strain rate on the fracture behaviour of skin. J Biosci. 1994;19(3):307-313.
- [CrossRef] [Google Scholar]
- Endolymphatic hydrops: mechanical causes of hearing loss. Arch Otorhinolaryngol. 1976;212(4):293-299.
- [CrossRef] [PubMed] [Google Scholar]
- Micro-lesions in Reissner's membrane evoked by acute hydrops. Audiol Neurotol. 2003;8(2):59-69.
- [CrossRef] [PubMed] [Google Scholar]
- Viscoelastic properties of isolated collagen fibrils. Biophys J. 2011;100(12):3008-3015.
- [CrossRef] [PubMed] [Google Scholar]
- The stiffness of living tissues and implications for tissue engineering. Nat Rev Mater. 2020;5:351-370.
- [CrossRef] [Google Scholar]








